Participate in Research
KCNQ2 Research In Action
Your Voice, Your Impact
Families hold the key to move research forward!
Before new treatments can be tested, researchers work behind the scenes in labs and models. This early phase helps scientists understand how the KCNQ2 gene affects brain cells, develop models (e.g., in cells or animals), and test ideas for what might help. Families may be invited to contribute by sharing medical histories, providing blood or tissue samples, or joining registries.
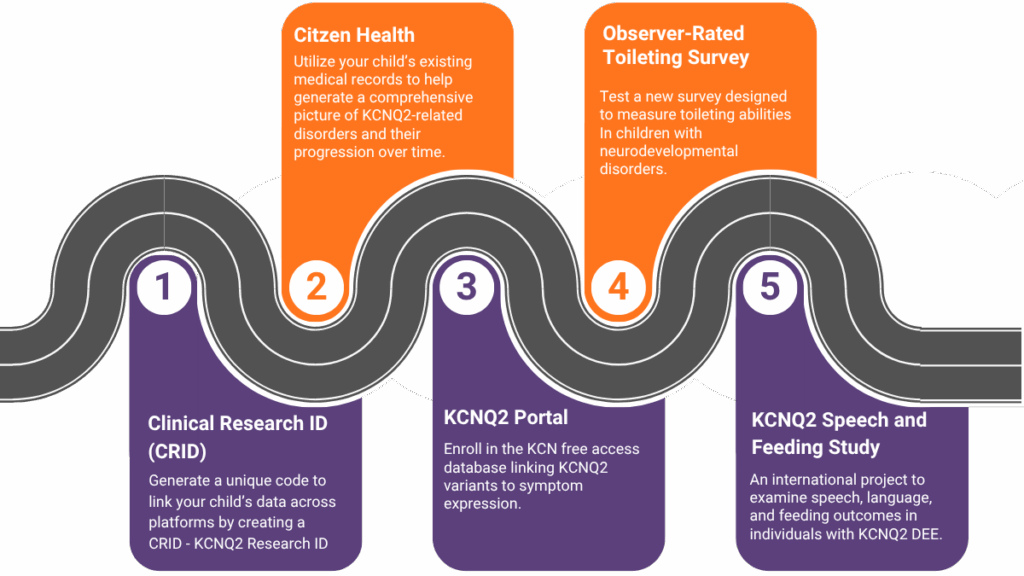
Your first step in participating in research and studies should be to create a CRID identifier (Clinical Research ID). Think of the CRID as your passport in the research world. It allows your data to “travel” safely between studies, hospitals, and scientists, enabling collaboration while protecting your identity. Patients can share their CRID identifier with investigators on research studies that support the CRID they are participating in. By sharing your CRID (KCNQ2 Research ID), researchers can then reuse, merge, and share your research data without using your personal identifiable information. This helps create a dataset for an individual vs. a variant. For more information, you can read this recent publication.
To create your CRID today, visit https://thecrid.org. You can watch the video below to see how quick it really is. It takes 2 minutes!

Introduction to the Clinical Research ID
Where To Begin???
Research Activites Currently Recruiting
Citizen Health
Digital Natural History Study- Citizen Health is a KCNQ2 digital natural history study that uses medical records to collect meaningful information on how a mutation in the KCNQ2 gene has affected a person’s life, and it includes medical record information across multiple hospitals and providers where a participant has been seen.
What is involved: One-time form to complete
Language: English
Who can participate: Anyone whose medical records are in English.
- For US families the document upload is automated.
- For those outside of the US with records in English, you will have to upload your documents manually.
How to participate: Enroll online at Citizen Health
KCNQ2 Portal and Phenotype
Observational Study: Clinical records often lack the depth needed to capture the full experience of neurodevelopmental disorders. By sharing your daily observations, you’ll help create a valuable dataset that highlights the nuanced, age-specific features of KCNQ2-related disorders, setting the stage for improvements in diagnosis and treatment.
What is involved: A 10-minute virtual consent; a 20-minute online survey; an optional virtual interview
Language: English
Who Can Participate: Any English speakers
How to Participate: Complete THIS FORM to participate
Observational Studies
 Observer Reported Toileting Abilities Survey (ORTAS) Validation Study
Observer Reported Toileting Abilities Survey (ORTAS) Validation Study
What: Toileting abilities are reported by families to be one of the most important aspects of daily living affected by neurodevelopmental disorders. This pilot study is for the purpose of testing a survey that will measure toileting abilities as an outcome measure.
What is involved: a 30-minute online survey consisting of questions about a person’s diagnosis, symptoms, treatments, and ability to toilet independently, or not. Any level of ability is welcome to participate.
Who Can Participate:
- Caregivers of a person (of any age) with a neurodevelopmental disorder (NDD)
- Caregivers of neurotypical children (ages 1-6) as controls
- Adults with a neurodevelopmental disorder (NDD)
How to Participate: email ORTAS@combinedbrain.org or visit www.combinedbrain.org for more information.
 KCNQ2 Speech and Feeding Study
KCNQ2 Speech and Feeding Study
What: An international project to examine speech, language, and feeding outcomes in individuals with KCNQ2 DEE. By improving our understanding of communication and feeding in KCNQ2, we hope to improve prognosis, better identify those who need support, and develop more targeted speech therapy practices.
What is involved: 1-hr virtual speech & feeding assessment; 2.5-hr online surveys
Language: English, French, German, Spanish, and Dutch
Who Can Participate: Those 6-months to adult with KCNQ2-DEE
How to Participate: Complete THIS FORM to show your interest.
Clinical Trials and Interventional Research Opportunities
The EMERALD Study
Praxis Precision Medicines
Phase: Phase 3 - Currently enrolling
Study medication: Reltrigine (Prax-562)
Type: Small Molecule
Who: For individuals with Developmental and Epileptic Encephalopathies (DEE)
Age: 2 through 65 years old
DEEp OCEAN Study
Lundbeck Pharmaceuticals
Phase: Phase 3 - Currently enrolling
Study medication: Bexicaserin (LP352)
Type: Small Molecule
Who: For individuals with Developmental and Epileptic Encephalopathies (DEE)
Age: 2 through 65 years old
LGS Discover Study
SK LifeScience Inc.
Phase: Phase 3 - Currently enrolling
Study medication: Carisbamte
Type: Small Molecule
Who: For individuals with a Lennox-Gastaut Syndrome diagnosis
Age: 4 through 55 years old
STARS Study
UCB / Parexel
Phase: Phase 3 - Currently enrolling
Study medication: Staccati Akorazikan (EP0162)
Type: Small Molecule
Who: For individuals with prolonged seizures lasting more than 3 minutes
Age: 12 years of age or older
X-TOLE2/X-TOLE3 Studies
Xenon Pharmaceuticals Inc
Phase: Phase 3 - Currently enrolling
Study medication: Azetukalner (XEN 1101)
Type: Small Molecule
Who: Adults with focal-onset seizures (FOS) or primary generalized tonic-clonic seizures (PGTCS)
Age: 18 years of age or older
X-ACKT Study
Xenon Pharmaceuticals Inc
Phase: Phase 3 - Currently enrolling
Study medication: Azetukalner (XEN 1101)
Type: Small Molecule
Who: Have experienced primary generalized tonic-clonic seizures (PGTCS) for at least 1 year
Age: 12 years of age or older
RISE
Biohaven Pharmaceuticals
Phase: Phase 2/3 - Currently enrolling
Study medication: BHV-7000
Type: Small Molecule
Who: Focal Onset Epilepsy
Age: 18 years to 75


